When it comes to lighting the road in front of your car or truck at night, most enthusiasts focus on light intensity instead of headlight bulb color. It’s straightforward to see the difference between yellowish light produced by incandescent halogen bulbs and the white/blue output of HID or LED bulbs. The science behind these light sources is interestingly similar to what professional car audio technicians measure to calibrate a digital signal processor in your mobile audio system. If you’re intrigued, read on, and we’ll explain in detail.
How Light Works
There have been many detailed scientific dissertations on how light works. These papers explain the electron and sub-electron concepts that allow us to see objects. In short, light is made of photons. Photons are packets of electrons that have been released from atoms. These packets of photons have energy and momentum but have no mass. This means you can shine a light at an object to illuminate it, but the energy from the light source doesn’t make the object heavier.
If we excite a group of atoms, the negatively charged electrons that orbit the nucleus absorb that energy. As more energy is added to an atom, the electrons circle faster and farther away from the center. When the energy source (electricity or heat) is removed, the electrons snap back to their original orbit path but release that added energy in the form of photons. Under specific conditions, the photons that are released produce visible light. If you studied electrical theory in high school, you’d recognize this pattern as similar to how electricity works. The only difference is that electricity involves electrons jumping from one atom to another to transfer energy.
When the light photons escape from an atom, they can have varying energy levels depending on the electron’s position when it leaves the atom. You can think of this as the photons having a specific resonant frequency. As a result, different types of atoms release photons of different wavelengths. The result is differently colored light sources.
Light and Color
We know that light sources have different colors. An incandescent bulb gives off a very different kind of light than a fluorescent bulb, a gas-discharge arc lamp (high-intensity discharge or HID) or a light-emitting diode (LED). Some light sources appear yellow, while others are white or blue. How these light sources illuminate objects can make them look very different.
Let’s take a giant step sideways. You’ve seen plenty of rainbows, but do you know what turns the supposedly white light from the sun into a color pattern that shifts from violet through to blue, green, yellow, orange and red? Water molecules refract the light from the sun. Because white light is made up of many different wavelengths, and each is reflected at a different angle as it passes through the water molecules, the light is divided into its primary components. Sorry, I know. We got all technical again.

An expensive-for-its-size electronic device called a spectral illuminance analyzer or a spectrometer can analyze the frequency content of a light source. The spectrometer works precisely the same way that a real-time audio analyzer (RTA) looks at the amplitude of the different sound frequencies produced by an audio source. As you may have guessed, we’ve added one to the BestCarAudio.com lab.



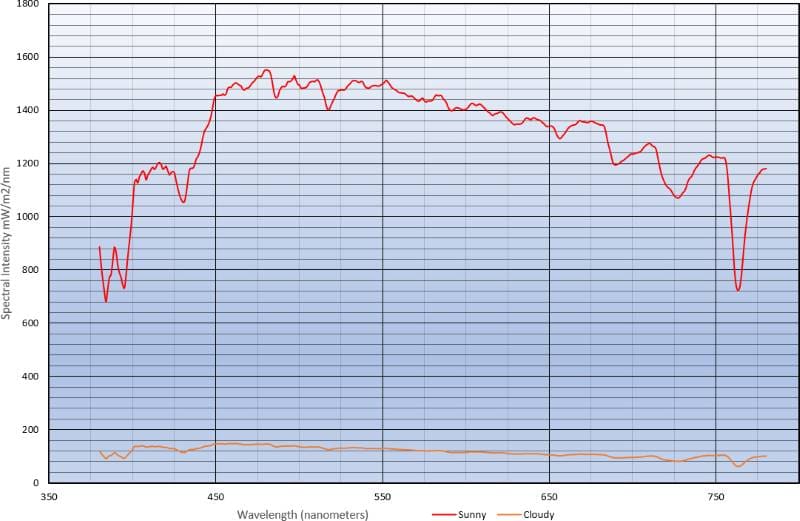
If you compare the two spectrographic measurements, you can see that the water vapor in the clouds is blocking increasing amounts of green, yellow, orange and red light. Unsurprisingly, we are left with a light source that makes everything look dull. This is because the water vapor in the air has quite literally filtered out the light energy that makes colors pop.
The software scales the measurement window to make it easy to see energy levels at different wavelengths. This is similar to the way our eyes or the iris and shutter on a camera work together to deliver a similar level of perceived brightness for a given lighting condition. The chart below shows both measurements overlaid, one on top of the other. You can see that the overall brightness level on a cloudy day is significantly lower.
The measured light level was 106,252 lux on a sunny day, whereas the cloudy day was only 9,069 lux. Converted to candlepower, the numbers are 9,874 and 843.
Headlight Bulb Color
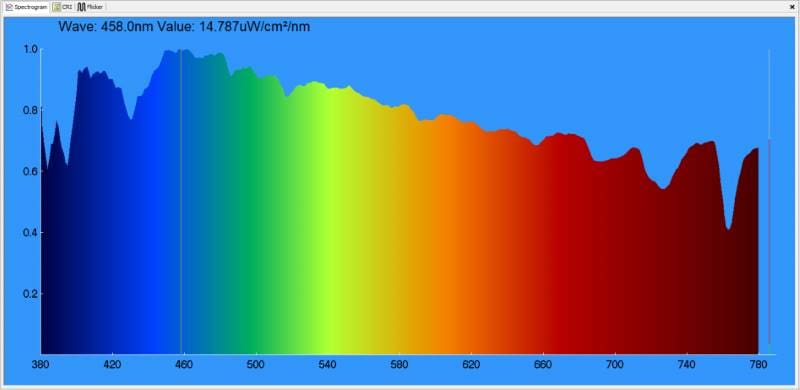
When it comes to the headlights on your car or truck, bulbs come in various colors for a variety of reasons. At the incandescent end of the spectrum, most have a yellowish look. With that said, halogen bulbs (which use iodine and bromine gas) have less yellow and produce more light output than old bulbs that use argon. Here’s the spectrographic analysis of a relatively simple halogen light bulb.
As you can see, there is a lot of energy in the red portion of the light spectrum produced by this bulb. To be clear, it’s not an amber bulb, though; we should find one of those and test it as well.

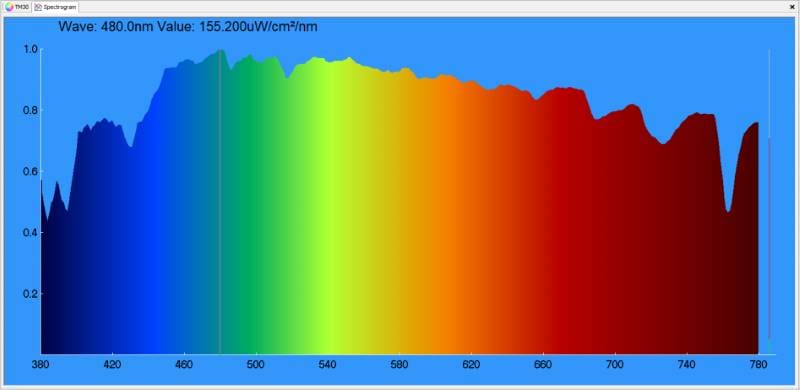
OK, we’re back from the hardware store with a pair of Sylvania 3057AK amber turn signal bulbs. The graph below shows their spectral energy.
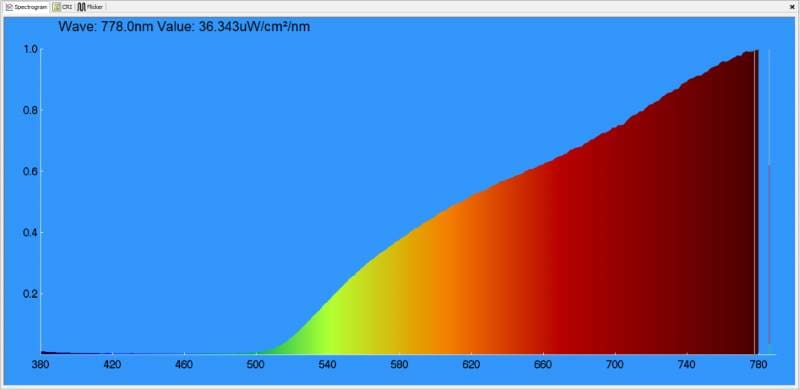
How we perceive the color of a light source is dependent on the frequency content of the energy coming from the bulb. Warm light will have more red energy, where a cool bulb will be bluer.
Color Temperature and Color Space
If you’ve ever shopped for HID headlight bulbs, you know their color is often described by a specific Kelvin value. For example, a yellow fog light bulb might be rated at 3,000 K, where a factory-installed HID or LED bulb might be a very pure white rated at 6,000 K. Those bulbs with a very blue tint are often up in the 8,000-10,000 K range.
Most people think these values are somewhat arbitrary, but the reality is, the light color can be measured with impressive accuracy using the right equipment. Our spectrometer can do this quickly and easily. The software will also plot the measurement on what’s called a color space chart. The chart outlines the level of red, green and blue in the light source and uses X and Y coordinates to describe the location on a chart. For our testing, we’ll use the CIE 1931 color space chart. The image below shows us where our measurement of the Wagner bulb falls.
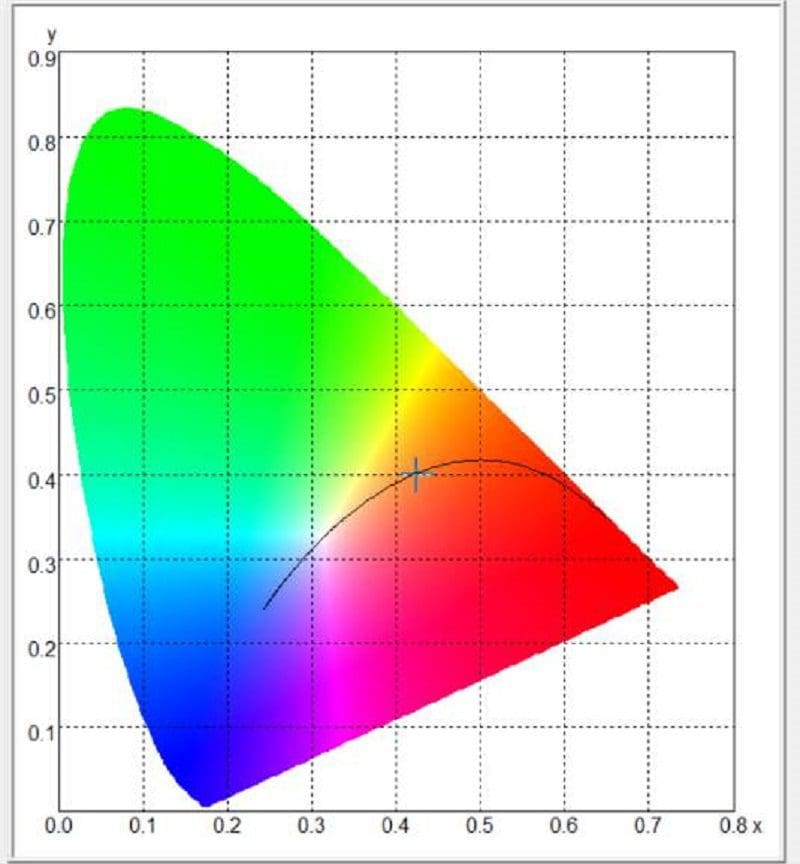
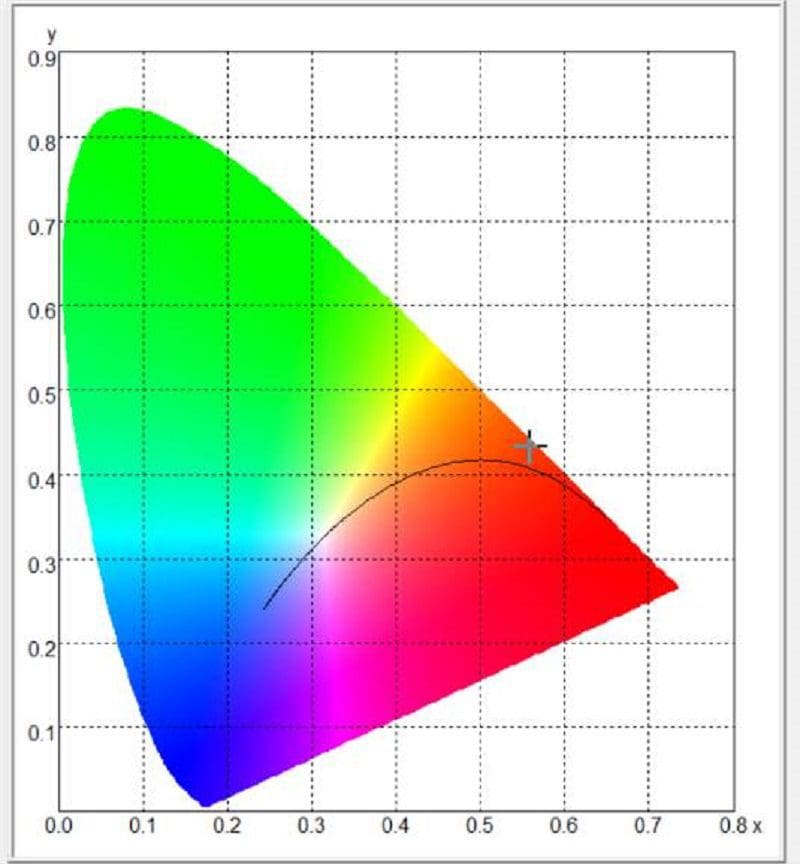
The software tells us the Wagner light source has a correlated color temperature of 3,174 kelvins. As mentioned, that’s considered a warm yellowish light. The amber Sylvania bulb has a color temperature of 1,857 and falls into the orange and red portion of the light spectrum.
White Light Isn’t Always Made Up Of All Frequencies
The last item we’ll touch on in this article is a bit of a tease toward some future content we are working on. If you’re reading this, then you’re likely looking at a computer or smartphone screen. The light created by that screen is made up of tiny red, green and blue pixels. The colors you see depend on the intensity of each of those pixels. If the screen is to be blue, then only the blue pixels will be illuminated. For violet, the red and blue will be turned on. Yellow is produced by red and green. You can easily see this pattern by looking at the CIE 1931 color space images above.
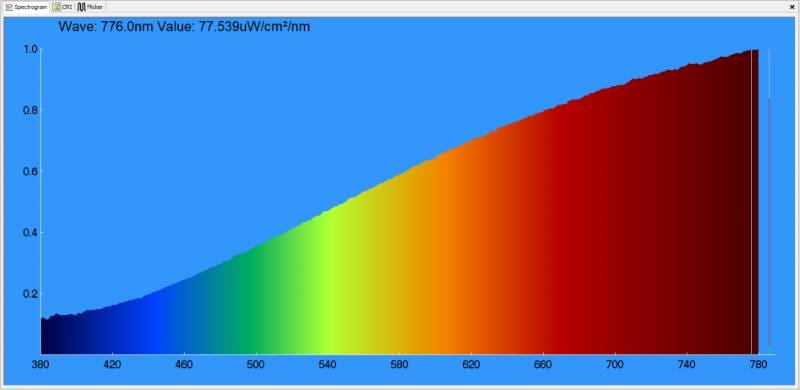
What might be surprising to some is that the perception of white can be made up of specific amounts of red, green and blue light. The chart below shows a measurement of the light produced by the laptop screen on which this article was created.
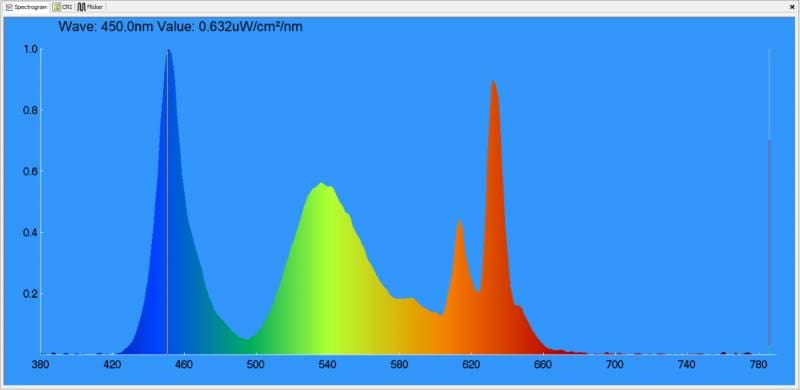
Behold! Our Dell XPS 13 laptop screen is perceived as white, yet it’s primarily red, very light green and mostly blue light. Here’s how the white light it produces measures on the CIE 1931 chart.
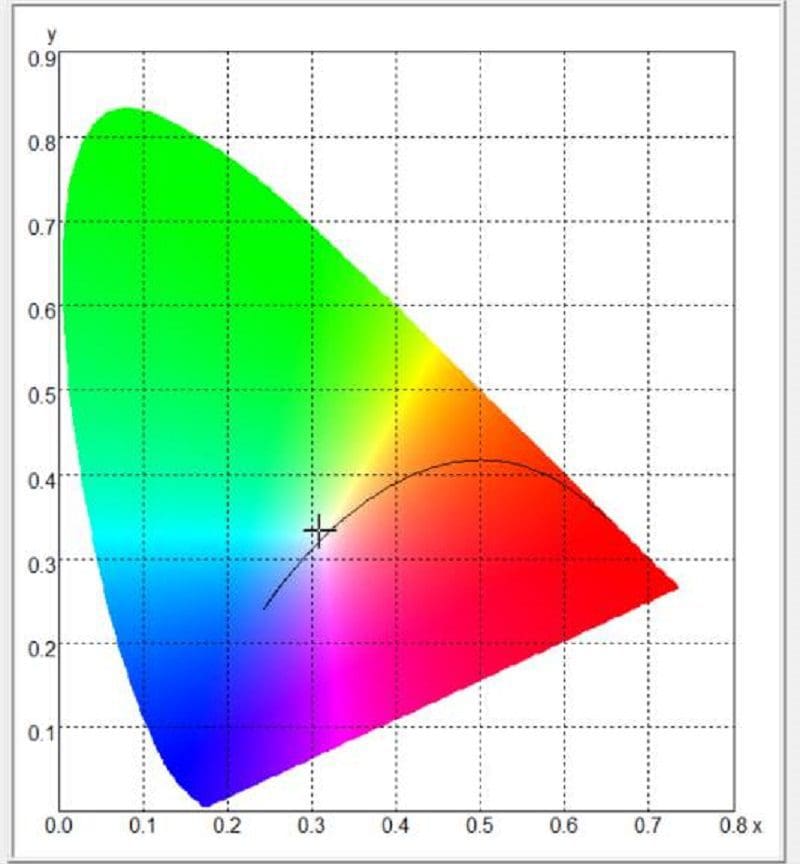
Our screen has a correlated color temperature of 6,662 K. If we were scoring it on even whiteness, that’d be an excellent result. But does this score mean it’s a perfect source of white light? Absolutely not! We’ll leave you to ponder that thought as we prepare the next few articles.
Lead-in Image: Thanks to Josh Matthews for sharing this photo of an Acura RSX equipped with decidedly blue headlights.

